The ATN Framework in Alzheimer’s Disease: Rethinking Diagnosis from Symptoms to Biology
 For decades, Alzheimer’s disease was diagnosed the way sailors once navigated—by observing the surface and inferring what lay beneath. Memory loss, disorientation, executive dysfunction, and behavioral changes formed the visible constellation. By the time the diagnosis was clear, the disease had often been silently active for years, sometimes decades.
For decades, Alzheimer’s disease was diagnosed the way sailors once navigated—by observing the surface and inferring what lay beneath. Memory loss, disorientation, executive dysfunction, and behavioral changes formed the visible constellation. By the time the diagnosis was clear, the disease had often been silently active for years, sometimes decades.
The ATN framework represents a quiet but profound shift in this thinking. It asks us to stop guessing from symptoms alone and instead look directly at the biological processes unfolding in the brain. In doing so, it reframes Alzheimer’s not as a clinical syndrome, but as a biologically defined disease continuum.
Why the ATN framework was needed
Clinical diagnosis has always lagged behind pathology in Alzheimer’s disease. Autopsy studies consistently showed amyloid plaques and tau tangles in individuals who were cognitively normal during life. Conversely, many patients with dementia-like symptoms had minimal Alzheimer-type pathology.
This mismatch created three problems:
-
Late diagnosis – By the time dementia is evident, neuronal loss is often extensive and irreversible.
-
Heterogeneity – Not all dementias with memory loss are Alzheimer’s disease.
-
Therapeutic failure – Disease-modifying trials failed partly because treatment began too late or targeted the wrong biology.
The NIA–AA ATN framework was proposed to solve this by anchoring Alzheimer’s disease to measurable biomarkers, not just clinical appearance.
Understanding the ATN framework
ATN is an acronym representing three core biological processes:
-
A – Amyloid pathology
-
T – Tau pathology
-
N – Neurodegeneration or neuronal injury
Each component is assessed independently and classified as positive (+) or negative (–). Together, they create a biological fingerprint of the brain.
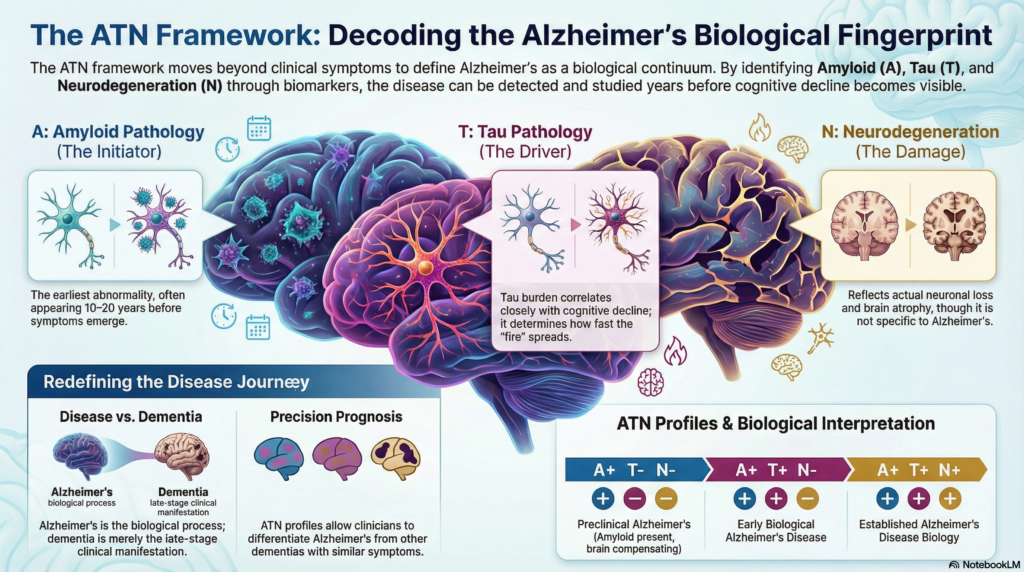
A: Amyloid pathology – the initiating event
Amyloid-β accumulation is believed to be the earliest detectable abnormality in Alzheimer’s disease. Importantly, amyloid deposition can begin 10–20 years before symptoms.
How amyloid is measured
-
CSF Aβ42 reduction or reduced Aβ42/Aβ40 ratio
-
Amyloid PET imaging
A positive A status indicates that Alzheimer’s pathology has biologically begun, even if cognition is intact.
Key insight:
Amyloid alone does not cause dementia. Many individuals die with amyloid plaques but without cognitive impairment. Amyloid is necessary, but not sufficient.
T: Tau pathology – the disease driver
Tau pathology reflects abnormal phosphorylation and aggregation of tau protein, particularly in medial temporal and associative cortices. Tau burden correlates much more closely with cognitive decline than amyloid.
How tau is measured
-
CSF phosphorylated tau (p-tau)
-
Tau PET imaging
A positive T status signals that Alzheimer’s disease has moved from silent pathology to active neurobiological injury.
Key insight:
If amyloid lights the fuse, tau determines how fast the fire spreads.
N: Neurodegeneration – downstream damage
Neurodegeneration reflects actual neuronal loss and synaptic dysfunction. Unlike amyloid and tau, N is not Alzheimer-specific.
How neurodegeneration is measured
-
MRI showing hippocampal or cortical atrophy
-
FDG-PET hypometabolism
-
CSF total tau elevation
A positive N status indicates that structural or functional brain damage has occurred.
Key insight:
N tells us about damage, not cause. Vascular dementia, frontotemporal dementia, and Alzheimer’s disease can all be N-positive.
Putting it together: ATN profiles
The true power of the ATN framework lies in combining these markers.
-
A–T–N–
Normal aging or non-neurodegenerative conditions -
A+T–N–
Preclinical Alzheimer’s disease (amyloid present, brain compensating) -
A+T+N–
Early biological Alzheimer’s disease -
A+T+N+
Established Alzheimer’s disease biology -
A–T+N+
Non-Alzheimer neurodegeneration (FTD, vascular, traumatic, etc.)
This explains why two patients with identical MMSE scores may have entirely different underlying diseases—and therefore different prognoses and treatment responses.
Alzheimer’s disease vs Alzheimer’s dementia
One of the most important conceptual shifts introduced by ATN is the separation of:
-
Alzheimer’s disease → a biological process
-
Alzheimer’s dementia → a clinical stage
A patient can have Alzheimer’s disease (A+T+) without dementia. Conversely, a patient can have dementia without Alzheimer’s disease.
This distinction is not academic—it is foundational for early intervention.
Clinical implications today
Although full ATN biomarker profiling is not yet feasible in many routine clinical settings (especially in resource-limited contexts), its influence is already visible:
-
Modern diagnostic criteria increasingly emphasize biomarkers
-
Disease-modifying therapies target amyloid and tau specifically
-
Clinical trials now enroll patients based on ATN status rather than symptoms alone
-
Prognostication is becoming biology-driven, not test-score driven
In practice, clinicians already apply ATN thinking implicitly when differentiating Alzheimer’s disease from vascular cognitive impairment, depression-related cognitive dysfunction, or frontotemporal syndromes.
Limitations and ethical considerations
The ATN framework is intentionally research-focused, not yet a standalone clinical diagnostic system. Important limitations remain:
-
Cost and availability of biomarkers
-
Psychological impact of identifying preclinical disease
-
Uncertainty about intervention thresholds
-
Cultural and healthcare-access disparities
Diagnosing a disease years before symptoms raises difficult ethical questions. Knowledge without actionable intervention can burden patients if not handled carefully.
The road ahead
ATN is not the final word. Future models will likely integrate:
-
Neuroinflammation
-
Synaptic biomarkers
-
Genetic risk (APOE and beyond)
-
Digital and cognitive phenotyping
Still, ATN has already done something transformative—it has forced us to see Alzheimer’s disease earlier, more clearly, and more honestly.
Closing reflections
The ATN framework teaches a humbling lesson:
by the time memory fails, the disease has already told its story in the brain—quietly, patiently, and long in advance.
For clinicians, researchers, and families alike, ATN offers a chance to shift from late recognition to early understanding. And in neurodegenerative disease, understanding is often the first real form of treatment.
Dr. Srinivas Rajkumar T
MD (AIIMS, New Delhi) | MBA (BITS Pilani)
Consultant Psychiatrist
Mind & Memory Clinic, Apollo Clinic, Velachery
Bridging clinical psychiatry, neuroscience, and early brain-based diagnosis.