Anti-Amyloid Monoclonal Antibodies in Alzheimer’s Disease
For many years, Alzheimer’s disease was treated symptom by symptom, long after the underlying brain changes had already done their damage. The idea of modifying the disease itself felt distant, almost theoretical. That changed in 2021, when aducanumab became the first anti-amyloid monoclonal antibody to receive FDA approval. The approval was controversial, but it marked an important moment—it showed that amyloid plaques in the human brain could actually be removed.
Since then, two newer antibodies, lecanemab and donanemab, have taken this idea further. They do not treat amyloid as a single target, nor do they claim to reverse dementia. Instead, they aim to intervene earlier, act on specific amyloid species, and slow progression in carefully selected patients. Together, these three drugs represent the first real attempt to shift Alzheimer’s treatment from symptomatic care to biology-based intervention.
Understanding what each of these antibodies does—and where their limits lie—is essential. They are not interchangeable, they are not cures, and they do not suit every patient. But they have fundamentally changed how we think about Alzheimer’s disease and its treatment.
The three major anti-amyloid monoclonal antibodies
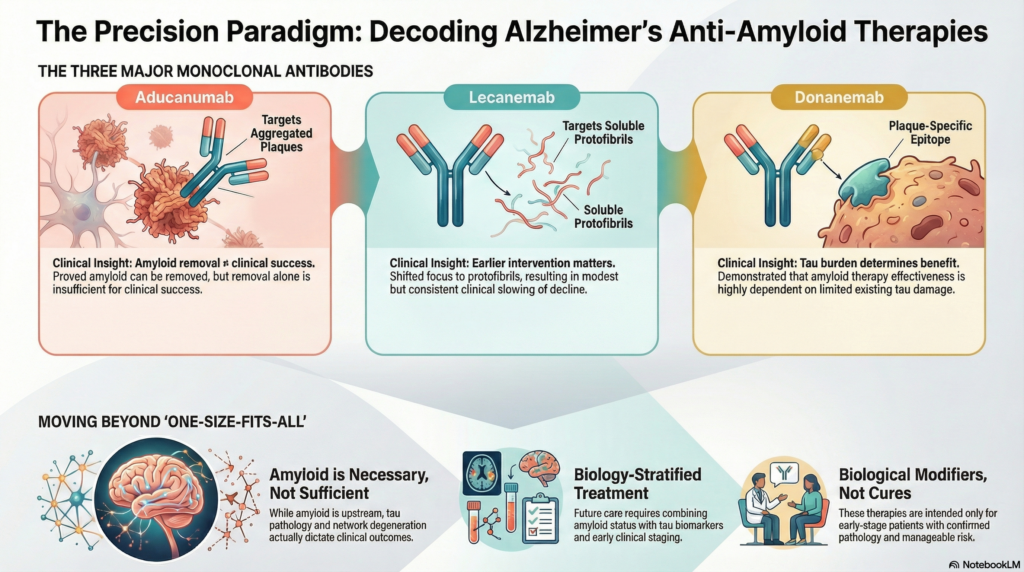
1. Aducanumab
Target: Aggregated amyloid-β (plaques and oligomers)
Mechanism:
Aducanumab selectively binds aggregated forms of amyloid-β and promotes plaque clearance via microglial-mediated phagocytosis.
Key facts:
-
First FDA-approved anti-amyloid antibody (accelerated approval, 2021)
-
Approval based on amyloid reduction, not unequivocal clinical benefit
-
High rates of ARIA (amyloid-related imaging abnormalities)
-
Sparked global debate on surrogate endpoints vs meaningful outcomes
Clinical takeaway:
Aducanumab proved amyloid can be removed—but also showed that removal alone is not enough.
2. Lecanemab
Target: Soluble amyloid-β protofibrils
Mechanism:
Unlike aducanumab, lecanemab preferentially targets protofibrillar amyloid, thought to be more neurotoxic and earlier in the disease cascade.
Key facts:
-
Demonstrated modest but consistent clinical slowing of decline
-
Better correlation between amyloid reduction and cognition
-
Lower ARIA rates compared to aducanumab (but not negligible)
-
Represents a shift toward earlier-stage intervention
Clinical takeaway:
Lecanemab marked the transition from “biological plausibility” to measurable clinical relevance, albeit modest.
3. Donanemab
Target: N-terminal pyroglutamate-modified amyloid-β (Aβp3-42)
Mechanism:
Donanemab binds a specific, highly aggregated plaque epitope, allowing aggressive plaque clearance.
Key facts:
-
Rapid amyloid clearance on PET imaging
-
Trials designed with treatment stopping rules once amyloid normalizes
-
Clinical benefit most evident in low-to-intermediate tau burden patients
-
Reinforces the importance of tau staging before amyloid therapy
Clinical takeaway:
Donanemab teaches a critical lesson: amyloid therapy works best when tau damage is still limited.
Why correct naming reflects deeper understanding
Confusing names often mirrors a deeper conceptual blur—treating all amyloid antibodies as interchangeable. They are not.
| Antibody | Primary Target | Clinical Insight |
|---|---|---|
| Aducanumab | Aggregated plaques | Amyloid removal ≠ clinical success |
| Lecanemab | Protofibrils | Earlier intervention matters |
| Donanemab | Plaque-specific epitope | Tau burden determines benefit |
Each antibody represents a different hypothesis about where amyloid matters most.
The bigger picture: amyloid is necessary, not sufficient
These therapies confirm three truths:
-
Amyloid is upstream in Alzheimer’s biology
-
Amyloid removal alone produces limited cognitive benefit
-
Tau pathology and network degeneration dictate outcomes
This is why modern trials increasingly combine:
-
Amyloid status
-
Tau biomarkers (PET or plasma p-tau)
-
Early clinical staging
The future is biology-stratified treatment, not one-size-fits-all infusions.
Practical clinical implications
Anti-amyloid monoclonal antibodies are:
-
Not cures
-
Not for advanced dementia
-
Not standalone therapies
They are best viewed as biological modifiers—slowing disease in carefully selected, early-stage patients with confirmed amyloid pathology and manageable risk profiles.
Dr. Srinivas Rajkumar T, MD (AIIMS, New Delhi), DNB, MBA (BITS Pilani)
Consultant Psychiatrist & Neurofeedback Specialist
Mind & Memory Clinic, Apollo Clinic Velachery (Opp. Phoenix Mall)
✉ srinivasaiims@gmail.com 📞 +91-8595155808