Antidepressant-Induced Sexual Dysfunction: Myths, Facts, and What the Evidence Actually Shows
 Sexual dysfunction is among the most consistently reported adverse effects of antidepressant treatment, yet it remains one of the least systematically discussed in routine clinical encounters. This gap between prevalence and conversation has real consequences: distress, relationship strain, and medication non-adherence.
Sexual dysfunction is among the most consistently reported adverse effects of antidepressant treatment, yet it remains one of the least systematically discussed in routine clinical encounters. This gap between prevalence and conversation has real consequences: distress, relationship strain, and medication non-adherence.
A fact-based examination is therefore not optional — it is clinically necessary.
How Common Is Antidepressant-Induced Sexual Dysfunction?
The headline number
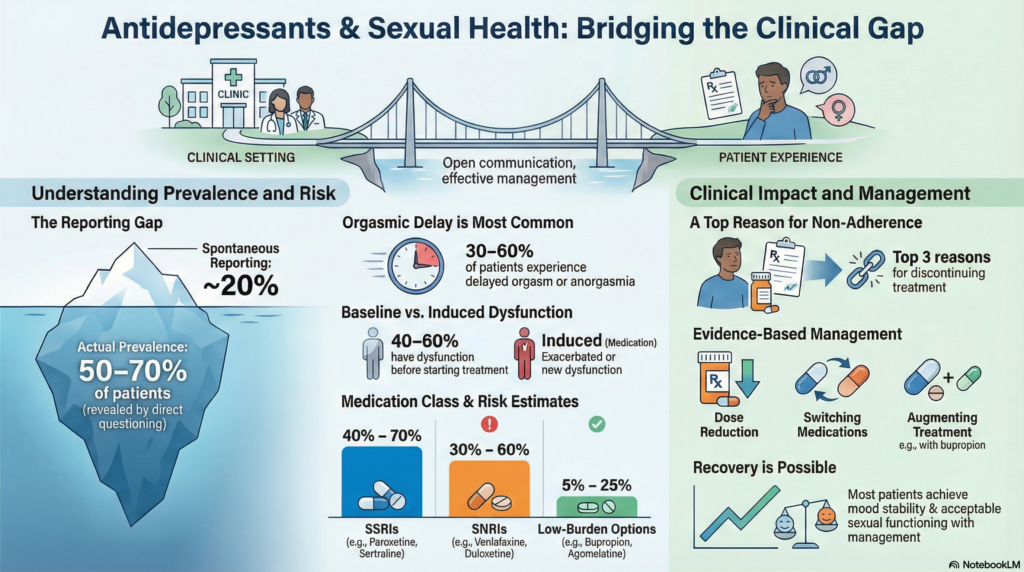
Across studies, 30–70% of patients treated with serotonergic antidepressants report some form of sexual dysfunction.
Why such a wide range?
Because prevalence varies depending on:
-
How directly patients are asked
-
Which phase of sexual response is assessed
-
Duration of treatment
-
Whether baseline sexual function was documented
What happens when patients are actively questioned?
When clinicians use structured sexual side-effect questionnaires, reported prevalence rises dramatically.
-
Spontaneous reporting: ~15–20%
-
Direct questioning: ~50–70%
This difference alone explains why many clinicians underestimate the problem.
Which Antidepressants Are Most Commonly Implicated?
SSRIs (Selective Serotonin Reuptake Inhibitors)
Large observational and trial data suggest:
-
Overall sexual dysfunction: 40–70%
-
Anorgasmia or delayed orgasm: 30–60%
-
Reduced libido: 25–50%
-
Erectile dysfunction (men): 20–40%
Among SSRIs:
-
Paroxetine consistently shows the highest rates
-
Fluoxetine and sertraline fall in the mid-range
-
Escitalopram shows somewhat lower but still significant rates
SNRIs (Serotonin–Norepinephrine Reuptake Inhibitors)
-
Venlafaxine: sexual dysfunction in ~40–60%
-
Duloxetine: ~30–50%
SNRIs tend to resemble SSRIs at higher doses due to increased serotonergic dominance.
Antidepressants With Lower Sexual Side-Effect Burden
Evidence consistently shows significantly lower rates with the following:
-
Bupropion:
-
Sexual dysfunction: ~5–15%
-
In some studies, improvement in libido and orgasm reported
-
-
Mirtazapine:
-
Sexual dysfunction: ~10–25%
-
-
Agomelatine:
-
Rates comparable to placebo (~5–10%)
-
-
Vortioxetine:
-
Sexual dysfunction: ~15–25%
-
Lower rates of anorgasmia than SSRIs
-
This difference is not anecdotal — it is pharmacologically predictable.
Which Phase of Sexual Response Is Most Affected?
Sexual dysfunction is not a single entity.
Desire (Libido)
-
Affected in ~30–50%
-
Strongly influenced by:
-
Depression severity
-
Relationship factors
-
Dopaminergic suppression
-
Arousal
-
Erectile dysfunction in men: ~20–40%
-
Lubrication difficulties in women: ~20–35%
Orgasm
-
Most commonly affected phase
-
Delayed ejaculation or anorgasmia in:
-
Men: ~40–60%
-
Women: ~30–50%
-
This pattern is classic for serotonergic medications.
Myth vs Reality: Is the Medication Always the Cause?
Baseline sexual dysfunction in depression
Before treatment even begins:
-
40–60% of patients with major depression already report sexual dysfunction
-
Libido loss is often an early symptom of depression itself
When antidepressants are started, three scenarios may occur:
-
Sexual function improves as depression lifts
-
Sexual dysfunction persists unchanged
-
New or worsened sexual dysfunction emerges
Only the third category represents true antidepressant-induced sexual dysfunction — and this distinction is often missed.
Do Sexual Side Effects Improve Over Time?
Yes — in a substantial minority of patients.
-
Partial adaptation occurs in ~20–30% over 8–12 weeks
-
Orgasmic delay is more persistent than libido changes
-
Persistent dysfunction beyond 6 months is not the norm, but not negligible
Time alone should not be the only strategy, but it is often part of the picture.
Post-SSRI Sexual Dysfunction (PSSD): What Does the Evidence Say?
PSSD refers to persistent sexual dysfunction after discontinuation of SSRIs.
What we know:
-
Reported, but rare relative to SSRI exposure
-
True prevalence is unknown
-
No definitive biological marker yet identified
What careful reviews suggest:
-
Likely affects a small subset of exposed individuals
-
Over-representation in online communities may inflate perceived risk
Ethically sound practice acknowledges:
-
Patient experience
-
Scientific uncertainty
-
Absence of evidence ≠ evidence of absence
But also avoids alarmist generalisation.
Why Sexual Side Effects Are Clinically Important
Sexual dysfunction is one of the strongest predictors of non-adherence.
Studies show:
-
Up to 30–40% of patients discontinue antidepressants prematurely
-
Sexual side effects rank among the top three reasons for discontinuation
This makes sexual side-effect management not optional, but central to treatment success.
Evidence-Based Management Strategies
Clinical studies support several approaches:
-
Dose reduction when feasible
-
Switching to lower-risk antidepressants
-
Augmentation with bupropion (improvement reported in ~50–70% in some trials)
-
Switching from SSRI to vortioxetine or agomelatine
-
Treating comorbid conditions (diabetes, thyroid disease, hypogonadism)
-
Addressing relational and psychological contributors
Importantly, most patients can achieve both mood stability and acceptable sexual functioning.
What the Evidence Does Not Support
-
That sexual dysfunction is inevitable
-
That it is permanent for most patients
-
That antidepressants “damage” sexuality
-
That patients must choose between mental health and intimacy
These beliefs are not evidence-based.
A Clinically Honest Conclusion
Antidepressant-induced sexual dysfunction is:
-
Common
-
Predictable
-
Mechanistically explainable
-
Often manageable
The real failure is not the side effect — it is failing to anticipate, measure, and address it.
When clinicians ask directly and patients speak freely, outcomes improve — both emotionally and relationally.
About the Author
Dr. Srinivas Rajkumar T
MD (AIIMS), DNB, MBA
Consultant Psychiatrist & Neurofeedback Specialist
Dr. Srinivas Rajkumar has a focused clinical interest in psychopharmacology, antidepressant side-effect management, and preserving quality of life in long-term psychiatric treatment.
Mind & Memory Clinic
Apollo Clinic Velachery (Opp. Phoenix Mall), Chennai
📞 +91-8595155808
✉ srinivasaiims@gmail.com