Methylphenidate vs Atomoxetine on QEEG: What Brain Waves Tell Us About How ADHD Medications Really Work
 Quantitative EEG (QEEG) has long occupied an uncomfortable middle ground in ADHD—too physiological to ignore, yet often dismissed as nonspecific. Still, when used longitudinally, QEEG offers something clinical rating scales cannot: a window into how medications alter brain state rather than whether symptoms improve.
Quantitative EEG (QEEG) has long occupied an uncomfortable middle ground in ADHD—too physiological to ignore, yet often dismissed as nonspecific. Still, when used longitudinally, QEEG offers something clinical rating scales cannot: a window into how medications alter brain state rather than whether symptoms improve.
Recent work comparing methylphenidate (MPH) and atomoxetine (ATX) provides an instructive example. These two drugs are often grouped together as “ADHD medications,” yet their neurophysiological fingerprints are not identical. When examined through QEEG, subtle but meaningful differences emerge—especially in frontal networks related to arousal and state regulation.
Why Compare MPH and Atomoxetine Using QEEG?
MPH (a dopamine–noradrenaline reuptake inhibitor) and ATX (a selective noradrenaline reuptake inhibitor) differ pharmacologically, clinically, and temporally:
-
MPH acts rapidly and prominently on phasic arousal and reward-related drive
-
ATX has slower onset and is often described as “stabilizing” rather than activating
If ADHD is increasingly understood as a disorder of arousal and effort regulation, QEEG should, in principle, reflect these differences.
The Core Comparative Study
A direct head-to-head QEEG comparison was published by Tokmakci et al. (2018) in Translational Neuroscience.
Study design (high relevance, modest scale)
-
40 participants:
-
ADHD + MPH (n = 10)
-
ADHD + ATX (n = 10)
-
Healthy controls (n = 20)
-
-
Ages: children/adolescents
-
Treatment duration: ~3 months at optimal dose
-
EEG: 19-channel resting EEG
-
Analysis: absolute band power + QEEG ratios
(delta/beta, delta/alpha, theta/alpha, theta/beta, theta/delta)
Despite its small sample, this study remains one of the few controlled MPH vs ATX QEEG comparisons available.
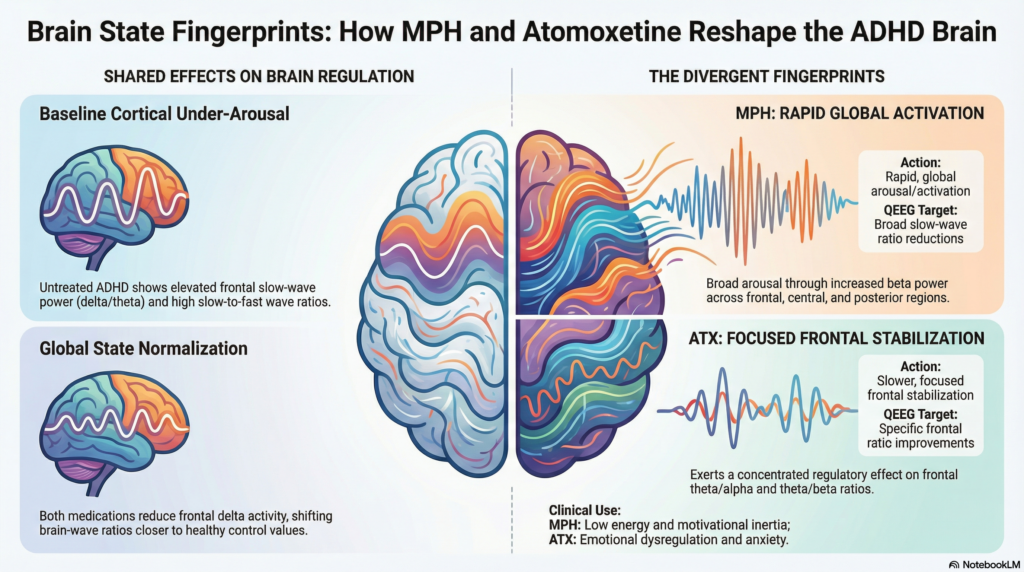
Baseline ADHD QEEG Pattern (Before Treatment)
Consistent with earlier literature, untreated ADHD showed:
-
Increased delta, theta, and alpha power
-
Predominantly frontal and fronto-central involvement
-
Elevated slow-to-fast wave ratios, particularly delta/beta and theta/beta
This aligns with the long-standing concept of cortical under-arousal rather than focal executive dysfunction.
Shared Effects of MPH and Atomoxetine
Both medications produced measurable QEEG changes over time.
Common findings
-
Reduction in frontal slow-wave activity, especially delta
-
Shift of key ratios toward control values, rather than complete normalization
-
Significant changes in:
-
Fz–Cz delta/beta ratio
-
C4–P4 delta/alpha ratio
-
These shared markers suggest that both drugs improve global brain state regulation, not merely “attention.”
Where MPH and Atomoxetine Diverge
Methylphenidate
-
Produces:
-
Reduction in slow-wave ratios across frontal, central, and posterior regions
-
Beta power increases, particularly parietal–occipital
-
-
Interpreted as:
-
Rapid enhancement of arousal and task readiness
-
Less regionally specific, more “global activation”
-
This fits MPH’s clinical profile: fast onset, noticeable activation, strong effects on motivation and persistence.
Atomoxetine
-
Demonstrated:
-
More statistically significant changes in frontal QEEG ratios
-
Stronger effects on:
-
theta/alpha
-
theta/beta
-
delta/alpha ratios
-
-
-
Frontal dominance was the authors’ central conclusion.
In plain terms, ATX appeared to exert a more focused frontal regulatory effect, consistent with its slower, steadier clinical action and emphasis on sustained state control rather than immediate stimulation.
How Do These Findings Fit With Other EEG Studies?
Although direct MPH–ATX comparisons are rare, related studies help contextualize the results.
-
Clarke et al. repeatedly demonstrated that stimulant treatment reduces theta and theta/beta ratio, particularly in responders.
-
Loo & Makeig highlighted that stimulant-related EEG changes reflect state normalization, not correction of a single ADHD “biomarker.”
-
Arns et al. showed that baseline theta/beta ratio predicts stimulant response—but less reliably for non-stimulants, supporting mechanistic divergence.
-
Barry et al. emphasized that ADHD EEG patterns reflect arousal dysregulation more than attentional circuitry per se.
Viewed together, ATX’s frontal QEEG prominence fits with a noradrenergic modulation of tonic alertness, while MPH produces a broader arousal–reward activation pattern.
Clinical Interpretation (Without Over-Promising QEEG)
QEEG should not be used to choose medication in isolation. But when used longitudinally, it can help us understand why a patient responds—or doesn’t.
Practical synthesis:
-
MPH
-
Faster, broader activation
-
Useful when low energy, psychomotor slowing, or motivational inertia dominate
-
-
ATX
-
Frontal stabilization, slower onset
-
May suit patients with emotional dysregulation, anxiety sensitivity, or rebound issues
-
Both improve ADHD symptoms—but via state regulation, not direct “attention enhancement.”
Where This Leaves Us Conceptually
QEEG data reinforces a growing shift in ADHD neuroscience:
-
ADHD is less about broken attention networks
-
More about unstable arousal, effort valuation, and persistence
-
Medications work by reshaping brain state, not sharpening cognition
MPH and ATX reach that endpoint by different physiological routes—and QEEG quietly captures that divergence.
References
-
Tokmakci M, Aldemir E, Yilmaz S, et al. Effects of methylphenidate and atomoxetine on quantitative EEG in children with attention-deficit/hyperactivity disorder. Transl Neurosci. 2018;9:231-240.
-
Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Res. 1998;81(1):19-29.
-
Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with ADHD. Clin Neurophysiol. 2003;114(3):464-472.
-
Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics. 2012;9(3):569-587.
-
Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374-383.
-
Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative EEG. Clin Neurophysiol. 2003;114(2):171-183.
Author Note
Dr. Srinivas Rajkumar T, MD (AIIMS), DNB, MBA (BITS Pilani)
Consultant Psychiatrist & Neurofeedback Specialist
Mind & Memory Clinic, Apollo Clinic Velachery (Opp. Phoenix Mall), Chennai
✉ srinivasaiims@gmail.com 📞 +91-8595155808
Clinical interests: ADHD phenotyping, QEEG-guided interventions, neurofeedback, and mechanism-based psychopharmacology.